Anna University CY6151 Engineering Chemistry - I UNIT IV PHASE RULE AND ALLOYS Chemical reactions are of two types 1. Irreversible reaction homogeneous 2. Reversible reaction – it's of two types - homogeneous
- heterogeneous
B.heterogeneous reversible reaction --- its behaviour can be studied by PHASE RULE given by Willard Gibbs (1874). Phase rule The number of degree of freedom (F) of the system is related to number of components (C) and number of phases (P) by the following phase rule equation. F = C-P+2 Explanation or meaning of terms 1. Phase (P) Any homogeneous physically distinct and mechanically separable portion of a system which is separated from other parts of the system by definite boundaries. a. Gaseous phase All gases are completely miscible and there is no boundary between one gas and the other. For example: air – single phase b.Liquid phase It depends on the number of liquids present and their miscibilities. i. If two liquids are immiscible, they will form three separate phases two liquid phase and one vapour phase. For example: benzene-water. ii. If tow liquids are miscible, they will form one liquid phase and one vapour phase. For example: alcohol – water. C .Solid phase Every solid constitutes a separate phase For example: (i) Water system ------- three phases (ii) Rhombic sulphur (s) à monoclinic sulphur (s) ----- two phase iii) Sugar solution in water ----- one phase iv) CuSO4.5H2O(s)  CuSO4.3H2O(s) + 2H2O(g) ---- three phases. CuSO4.3H2O(s) + 2H2O(g) ---- three phases. 2. Component (C) "The smallest number of independently variable constituents, by means of which the composition of each phase can be expressed in the form of a chemical equation". For example: i) Water system ---- one component ( H2O ) ii) An aqueous system of NaCl --- two component ( NaCl , H2O ) iii) PCl5(s) ![clip_image003[1] clip_image003[1]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEi_nILRcKRsKdISLKzyLe34pIdnZntbdDYjB5Q4LBNN3tOBImbBHzsNT0blHVxjQ7TR9UWpcjGW-KYP6GRmdq_fRpYcUVAnm1zvSvTU-raZCtCotHWrMaatp9vlGAd4-9nYkqNEqLvlY38/?imgmax=800) PCl3 (l) + Cl2 (g) --- two component ,three phases PCl3 (l) + Cl2 (g) --- two component ,three phases iv) CuSO4.5H2O(s) ![clip_image003[2] clip_image003[2]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEi-VvIi_159hApd1_ktWMuBIl_x_iLCtqV2XvPUPxwqDxmcRN9EvW6-5aENqUtnDOZ9MzNJuJ0YgJPuSR53ep-7PDRJ8ueoUDS9niBsxmGEgTYo69RV7RepcBmjWcl_UbDyyhtq-3hJWN8/?imgmax=800) CuSO4.3H2O(s) + 2H2O(g) ---- three phases,two component CuSO4.3H2O(s) + 2H2O(g) ---- three phases,two component 3. Degree of freedom(F) "The minimum number of independent variable factors such as temperature, pressure and concentration, which much be fixed in order to define the system completely". i) Water system 
F = Non variant (or) zero variant 
F = univariant (one) iii) For a gaseous mixture of N2 and H2, we must state both the pressure and temperature. Hence,the system is bivariant. PHASE DIAGRAM: Phase diagram is a graph obtained by plotting one degree of freedom against another. Types of phase diagrams (i)P-T Diagram : used for one component system (ii) T-C Diagram : used for two component system APPLICATIONS OF PHASE RULE TO ONE COMPONENT SYSTEM The water system: Water exists in three possible phases namely solid, liquid and vapour. Hence there can be three forms of equilibria. 
Each of the above equilibrium involves two phases. The phase diagram for the water system is shown in the figure. This phase diagram contains curves, areas, and triple. 
(i)Curve OA The curve OA is called vaporisation curve, it represents the equilibrium between water and vapour. At any point on the curve the following equilibrium will exist. 
The degree of freedom of the system is one, i.e, univariant. This is predicted by the phase rule. F=C-P+2; F=1-2+2; F=1 This equilibrium (i.e. Line OA) will extend up to the critical temperature (347o C). Beyond the critical temperature the equilibrium will disappear only water vapour will exist. (ii) Curve OB The curve OB is called sublimation curve of ice, it represents the equilibrium between ice and vapour. At any point on the curve the following equilibrium will exist. 
The degree of freedom of the system is one, i.e. univariant. This is predicted by the phase rule. F = C – P + 2; F = 1-2=2 ; F=1 This equilibrium (line OB) will extend up to the absolute zero (-273o C), where no vapour can be present and only ice will exist. iii) Curve OC The curve OC is called melting point curve of ice, it represents the equilibrium between the ice and water. At any point on the curve the following equilibrium will exist. 
The curve OC is slightly inclined towards pressure axis. This shows that melting point of ice decreases with increase of pressure. The degree of freedom of the system is one i.e., univariant. iv) point O (triple point) The three curves OA ,OB ,OC meet at a point "O" ,where three phases namely solid ,liquid and vapour are simultaneously at equilibrium . This point is called triple point, at this point the following equilibrium will exist. 
The degree of freedom of the system is zero i.e., nonvariant.This is predicted by the phase rule. F=C-P+2; F=1-3+2=0 Temperature and pressure at the point "O" are 0.0075 oC and 4.58 mm respectively. (v) Curve OB': Metastable equilibrium The curve OB' is called vapour pressure curve of the super-cool water or metastable equilibrium where the following equilibrium will exist. Super-cool water  vapour vapour Sometimes water can be cooled below OoC without the formation of ice, this water is called super –cooled water. Super cooled water is unstable and it can be converted in to solid by seeding or by slight disturbance. vi) Areas Area AOC, BOC, AOB represents water, ice and vapour respectively .The degree of the freedom of the system is two.i.e. Bivariant. This is predicted by the phase rule F=C-P=2; F=1-1+2; F=2 Two component alloy system or multi component equilibria Reduced phase rule or condensed system The system in which only the solid and liquid are considered and the gas phase is ignored is called a condensed system.since pressure kept constant, the phase rule becomes F' = C – P + 1 This equation is called reduced phase rule. Classification of two component system Based on the solubility and reactive ablity, the two component systems are classified in to three types. 1. Simple eutectic formation - A binary system consisting of two substances, which are completely miscible in the liquid state, but completely immiscible in the solid state, is known as eutectic (easy melt) system. They do not react chemically. Of the different mixtures of the two substances, the mixture having the lowest melting point is known as the eutectic mixture. 2. a) formation of compound with congruent melting point b) Formation of compound with incongruent melting point 3. Formation of solid solution Thermal analysis or cooling curve Thermal analysis is a method involving a study of the cooling curves of various compositions of a system during solidification. The form of the cooling curve indicates the composition of the solid. Ex: 1. Cooling curve of a pure solid. Ex: 2. Cooling curve of a mixture A + B. A cooling curve is a line graph that represents the change of phase of matter, typically from a gas to a solid or a liquid to a solid. The independent variable (X-axis) is time and the dependent variable (Y-axis) is temperature. Below is an example of a cooling curve. 
The initial point of the graph is the starting temperature of the matter, here noted as the "pouring temperature". When the phase change occurs there is a "thermal arrest", that is the temperature stays constant. This is because the matter has more internal energy as a liquid or gas than in the state that it is cooling to. The amount of energy required for a phase change is known as latent heat. The "cooling rate" is the slope of the cooling curve at any point. A Pure substance in the fused or liquid state is allowed to cool slowly. The temperature is noted at different times.when represented graphically the rate of cooling will be a continuous from 'a' to 'b'. When the freezing point is reached and solid making its appearance there will be a break in the continuity of the cooling curve.The temperature will thereafter remain constant until the liquid is completely solidified.Thereafter the fall in temperature wil again become continuous. 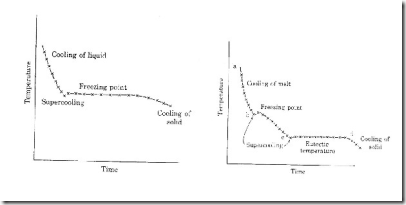
a. Cooling curve of a pure substances b. Cooling curve of a mixture If a mixture of two solids in the fused state is cooled slowly we get a cooling curve . Here also first a continuous coling curve will be obtained as long as the mixture is in the liquid state . When a solid phase begins to form there will be a break in the cooling curve .But the temperature will not remain constant unlike in the case of cooling of a purified substance.The temperature will decrease continuously but at a different rate.The fall of temperature will continue till the mixture forms a eutectic and the eutectic point is reached. The temperature will thereafter remain constant until solidification is complete . Thereafter the fall of temperature will become uniform ,but the rate of fall will be different from that for a pure substance. Uses of cooling curves i) Percentage purity of the compounds can be noted from the cooling curve. ii) The behaviour of the compounds can be clearly understood from the cooling curve. iii) The procedure of thermal analysis can be used to derive the phase diagram of any two component system. BINARY ALLOY SYSTEM OR THE SIMPLE EUTECTIC SYSTEM The Lead – Sliver system Since the system is studied at constant pressure,the vapour phase is ignorned and the condensed phase rule is rule is used. F I= C-P+1 The phase diagram of lead –sliver system is shown in the figure It contains lines,areas and the eutectic point. 
i) curve AO The curve AO is known as freezing point curve of sliver. Along the curve AO, solid Ag and the melt are in equilibrium. Solid <------------> Ag melt According to reduced phase rule F'=C-P+1 C=2 P=2 F'=1 The system is univariant ii) curve BO The curve BO is known as freezing point curve of lead . Along the curve BO, solid Pb and the melt are in equilibrium. Solid Pb <----------->melt According to reduced phase rule F'=C-P+1 C=2 P=2 F'=1 The system is univariant. iii) Point " O " (eutectic point) The curves AO and BO meet at point ' O ' at a temperature of 303 o C ,where the three phases are in equilibrium. Solid Pb + soild Ag <---------> melt According to reduced phase rule F'=C-P+1 C=2 P=3 F'=1 The system is non-variant. The point " O " is called eutectic point or eutectic temperature and is corresponding composition,97.4 % Pb and 2.6 % Ag ,is called eutectic composition.below this point the eutectic compound and the metal solidfy. iv) Areas The area above the line AOB has a single phase( molten Pb + Ag ). According to reduced phase rule F'=C-P+1 C=2 P=1 F'=2 The system is bi-variant. The area below the line AO ,OB and point "O" have two phases and hence the system is univariant. According to reduced phase rule F'=C-P+1 C=2 P=2 F'=1 The system is uni-variant. 
The process of raising the relative proportion of Ag in the alloy is known as pattinson's process. The Pattinson process was patented in 1833. It depended on well-known material properties; essentially that lead and silver melt at different temperatures. The equipment consisted of a row of about 8-9 iron pots, which could be heated from below. Agentiferous lead was charged to the central pot and melted. This was then allowed to cool, as the lead solidified, it was skimmed off and moved to the next pot in one direction, and the remaining metal was then transferred to the next pot in the opposite direction. The process was repeated in the pots successively, and resulted in lead accumulating in the pot at one end and silver in that at the other. The process was economic for lead containing at least 250 grams of silver per ton. Uses of eutectic system 1.suitable alloy composition can be predicted with the help of eutectic systems. 2.eutectic systems are used in preparing solders ,used for joining two metal pieces together. Melting point It is the temperature at which the solid and liquid phases, having the same composition ,are in equilibrium. Solid A <------> solid B Eutectic point It is the temperature at which two solids and a liquid phase are in equilibrium . Solid A + solid B <-----> Liquid Triple point It is the temperature at which three phases are in equilibrium. Solid <---->liquid<-------> vapour By definition , All the eutectic points are melting points, but all the melting points need not be eutectic points. ll ly , all the eutectic points are triple points ,but all the triple points need not be eutectic points. Uses (or) merits of phase rule 1. It is a convenient method of classifying the equilibrium states in terms of phases ,components and degree of freedom. 2. It helps in deciding whether the given number of substances remain in equilibrium or not. Limitations of phase rule 1.phase rule can be applied for the systems in equilibrium. 2.only three variables like P,T & C are considered ,but not electrical, magnetic and gravitational forces. Definition ALLOYS An alloy is defined as "homogeneous solid solution of two or more different element one of which at least is essentially a metal". Alloy containing Hg as a constituent element are called amalgams. Properties of alloys 1) Alloy are harder less malleable and possess lower melting point than their component metals 2) Alloys possess low electrical conductivity 3) Alloys resist corrosion and the action of acids Importance or need of making alloys 1. To increase the hardness of the metal Example Gold and silver are soft metal they are alloyed with copper to make them hard 2. To lower the melting points of the metal Example Wood metal (an alloy of lead, bismuth, tin and cadmium) melts at 60.5⁰c which is far below the melting points of any of these constituent metals 3. To resist the corrosion of the metal Example Pure iron rested but when it is alloyed with carbon chromium (stainless steel) which resists corrosion 4. To modify chemical activity of the metal Example Sodium amalgam is less active than sodium but aluminium amalgam is more active than aluminium 5. To modify the colour of the metal Example Brass an alloy of copper (red) and size (silver-white) is white colour. 6. To get good casting of metal Example An alloy of lead with 5% tin and 2% antimony is used fro casting printing type due toits good casting property Functions (or) effects of alloying elements Addition of small amount of certain metals such as Ni, Cr, Mo, Mn, Si, v and Al impart special properties like hardness, tensile strength, resistance to corrosion and coefficient of expansion on steel. Such products are known as special steel or alloy steels Some important alloying element and their functions are given in table CLASSIFICATION (OR) TYPES OF ALLOYS 
PROPERTIES 1. High yield point & strength 2. Sufficient formability,ductility & weldability 3. Corrosion & abrasion resistant 4. Less distortion & cracking 5. High temperature strength IMPORTANT FERROUS ALLOYS (i)NICHROME Nichrome is an alloy of nickel & chromium COMPOSITION Nickel – 60% Chromium – 12% Iron – 26% Manganese – 2% PROPERTIES 1. Good resistance to oxidation & heat 2. High melting point & electrical resistance 3. Withstand heat up to 1000-1100⁰C USES 1. Used for making resistance coils,heating elements in stoves & electric irons 2. Used in making parts of boilers,steam lines stills,gas turbines,aero engine valves,retorts,annealing boxes. (ii)ALNICO Alnico is an alloy of aluminium-nickel-cobalt . COMPOSITION Aluminium – 8-12% Nickel – 14-28% Cobalt – 5-35% PROPERTIES 1. Excellent magnetic properties & high melting point 2. Magnetized to produce strong magnetic fields as high as 1500 gauss TYPES OF ALNICO ALLOYS Alnico alloys are of two types 1. ISOTROPIC ALNICO It is effectively magnetized in any direction 2.ANISOTROPIC ALNICO It possess preffered direction of magnetization. Anisotropic alnico possesses greater magnetic capacity in their preffered orientation than isotropic alnico. USES 1. Used as permanent magnets in motors,generators,radio speakers microphones,telephone receivers & galvanometers. (iii)STAINLESS STEELS (or)CORROSIOPN RESISTANT STEELS · These are alloy steels containing chromium together with other elements such as nickel,molybdenum,etc. · Chromium-16% or more · Carbon-0.3-1.5% PROPERTIES 1. Resist corrosion by atmospheric gases & also by other chemicals. 2. Protection against corrosion is due to the formation of dense, non- porous,tough film of chromium oxide at the metal surface. If the film cracks, it gets automatically healed up by atmospheric oxygen TYPES OF STAINLESS STEEL 

1. HEAT TREATABLE STAINLESS STEEL COMPOSITION Carbon-1.2% Chromium-less than 12-16% PROPERTIES Magnetic,tough & can be worked in cold condition USES 1. Can be used up to 800⁰C 2. Good resistant towards weather & water 3. In making surgical instruments,scissors,blades,etc. 2.HEAT TREATABLE STAINLESS STEEL PROPERTIES · Possess less strength at high temperature · Resistant to corrosion TYPES OF NON HEAT TREATABLE STAINLESS STEEL (a)MAGNETIC TYPE COMPOSITION Chromium-12-22% Carbon-0.35% PROPERTIES 1. Can be forged,rolled & machined 2. Resist corrosion USES Used in making chemical equipments& automobile parts. (b)NON MAGNETIC TYPE COMPOSITION Chromium-18-26% Nickel-8-21% Carbon-0.15% Total % of Cr & Ni is more than 23%. EXAMPLE:18/8 STAINLESS STEEL COMPOSITION: Chromium-18% Nickel-8% PROPERTIES 1. Resistance to corrosion. 2. Corrosion resistance is increased by adding molybdenum USES In making household utensils,sinks,dental & surgical instruments. NON FERROUS ALLOYS · Do not contain iron as one of the main constituent. · Main constituents are copper,aluminium,lead,tin,etc. PROPERTIES 1. Softness & good formability 2. Attractive (or) very good colours 3. Good electrical & magnetic properties 4. Low density & coefficient of friction 5. Corrosion resistance IMPORTANT NON FERROUS ALLOYS 1. COPPER ALLOYS (BRASS) Brass contains mainly copper & zinc PROPERTIES · Greater strength, durability & machinability · Lower melting points than Cu & Zn · Good corrosion resistance & water resistance property 2.BRONZE(COPPER ALLOY) Bronze contains copper & tin PROPERTIES · Lower melting point · Better heat & electrical conducting property · Non-oxidizing,corrosion resistance & water resistance property. 3.SOLDERS Solders are low- melting alloys of tin & lead PROPERTIES Solder is melted to join metallic surfaces ,especially in the fields of electronic & plumbing USES 1. Used in electrical industry 2. Alloy with 50% tin is general-purpose solder 3. For sealing automotive radiator cores. 4. As fuses for fire-extinguishing equipments,boiler plugs,etc. Heat treatment of alloys (steel) Heat treatment is defined as" the process of heating and cooling of solid steel article under carefully controlled condition". During heat treatment certain physical properties are altered without altering its chemical composition Objectives (or) purpose of heat treatment Heat treatment causes i. Improvement in magnetic and electrical properties ii. Refinement of grain structure iii. Removal of the imprisoned trapped gases iv. Removal of internal stress v. Improves fatique and corrosion resistance Types of heat treatment of alloys (steel) 1. Annealing Annealing means softening. This is done by heating the metal to high temperature followed by slow cooling in a furnace. Purpose of annealing i. It increases the machinability ii. It also removes the imprisoned gases Types of annealing Annealing can be done in two types i. Low temperature annealing (or) process annealing ii. High temperature annealing 9or) full annealing Low temperature annealing (or)process annealing It involves in heating steel to a temperature below the lower critical point followed by slow cooling Purpose 1. It improves mashinability by reliving the internal stress or internal strain 2. It increases ductility and shock resistance 3. It reduce hardness (i) High temperature annealing (or) fault annealing It involves in heating to a temperature about 30 to 50⁰C above the higher critical temperature and holding it at that temperature for sufficient time to allow the internal changes to take place and then cooled room temperature The approximate annealing temperature of various grades of carbon steel are 1. Mild steel=840-870⁰c 2. Medium carbon steel=780-840⁰c 3. High carbon steel=760-780⁰c Purpose 1. It increases the ductility and machinability 2. It makes the steel softer, together with an appreciable increases in its toughness 2.Hardening (or) quenching · It is the process of heating steel beyond the critical temperature and then suddenly cooling it either in oil or brine water or some other fluid. · The faster the rate of cooling harder will be the steel produced. · Medium and high carbon steel can be hardened but low carbon steel cannot hardened Purpose 1. It increases its resistance to wear ability ,to cut other metal and strength . 2. It increases abrasion resistance. 3. Used for making cutting tools. 3. TEMPERING · It is the process of heating the already hardened steel to a temperature lower than its own hardening temperature & then cooling it slowly. · The reheating controls the development of the final properties · Thus, (a)For retaining strength & hardness, reheating temperature should not exceed 400⁰C. (b) For developing better ductility & toughness, reheating temperature should be within 400-600⁰C. Purpose 1. It removes stress &strains that might have developed during quenching. 2. Increased toughness & ductility. 3. Used for cutting tools like blade,cutters etc. 4. NORMALISING It is the purpose of heating steel to a definite temperature (above its higher critical temperature) & allowing it to cool gradually in air. Purpose 1. Recovers homogeneity 2. Refines grains. 3. Removes internal stresses 4. Increases toughness 5. Used in engineering works NOTE: The difference between normalised & annealed steel are 1. A normaled steel will not be as soft as annealed steel. 2. Also normalizing takes much lesser time than annealing. 5.CARBURIZING · The mild steel article is taken in a cast iron box within containing small pieces of charcoal(carbon material). · It is heated to about 900 to 950⁰C & allow it for sufficient time,so that the carbon is absorbed to required depth . · The article is then allowed to cool slowly within the box itself. · The outer skin of the article is converted into high carbon steel containing about 0.8 to 1.2% carbon. Purpose To produce hard surface on steel article 6.NITRIDING Purpose · Nitriding is the process of heating the metal alloy in presence of ammonia to about 550⁰C. · The nitrogen (obtained by the dissociation of ammonia) combines with the surface of the alloy to form hard nitride. To get super-hard surface.  |
No comments:
Post a Comment